Toll Free Helpline (India): 1800 1234 070
Rest of World: +91-9810852116
Free Publication Certificate
Vol. 3, Issue 9 (2014)
Novel analytical method development and validation for the quantitative analysis of Efavirenz in bulk and pharmaceutical dosage forms by RP-HPLC
Author(s):
P. Ravisankar, G. Mounika, CH. Devadasu, G. Devala Rao
P. Ravisankar, G. Mounika, CH. Devadasu, G. Devala Rao
Abstract:
A convenient, simple, specific, accurate, precise, rapid, inexpensive isocratic Reversed Phase-High Performance Liquid Chromatography (RP-HPLC) method was developed and validated for the quantitative determination of Efavirenz in pharmaceutical tablet dosage forms. RP-HPLC method was developed by using Welchrom C18 Column (4.6 X 250 mm, 5µm), Shimadzu LC-20AT Prominence Liquid Chromatograph. The mobile phase composed of 10 mM Phosphate buffer (pH-3.0, adjusted with triethylamine): acetonitrile (50:50 v/v). The flow rate was set to 1.2 mL.min-1 with the responses measured at 246 nm using Shimadzu SPD-20A Prominence UV-Vis detector. The retention time of Efavirenz was found to be 9.563 minutes. Linearity was established for Efavirenz in the range of 2-10 µg.mL-1 with correlation coefficient 0.9999. The LOD and the LOQ were found to be 0.0183 μg.mL-1 and 0.0555 μg.mL-1 respectively. The amount of Efavirenz present in the formulation was found to be 99.82%. The validation of the developed method was carried out for specificity, linearity, precision, accuracy, robustness, limit of detection, limit of quantitation. The developed method can be used for routine quality control analysis of Efavirenz in pharmaceutical tablet dosage form.
A convenient, simple, specific, accurate, precise, rapid, inexpensive isocratic Reversed Phase-High Performance Liquid Chromatography (RP-HPLC) method was developed and validated for the quantitative determination of Efavirenz in pharmaceutical tablet dosage forms. RP-HPLC method was developed by using Welchrom C18 Column (4.6 X 250 mm, 5µm), Shimadzu LC-20AT Prominence Liquid Chromatograph. The mobile phase composed of 10 mM Phosphate buffer (pH-3.0, adjusted with triethylamine): acetonitrile (50:50 v/v). The flow rate was set to 1.2 mL.min-1 with the responses measured at 246 nm using Shimadzu SPD-20A Prominence UV-Vis detector. The retention time of Efavirenz was found to be 9.563 minutes. Linearity was established for Efavirenz in the range of 2-10 µg.mL-1 with correlation coefficient 0.9999. The LOD and the LOQ were found to be 0.0183 μg.mL-1 and 0.0555 μg.mL-1 respectively. The amount of Efavirenz present in the formulation was found to be 99.82%. The validation of the developed method was carried out for specificity, linearity, precision, accuracy, robustness, limit of detection, limit of quantitation. The developed method can be used for routine quality control analysis of Efavirenz in pharmaceutical tablet dosage form.
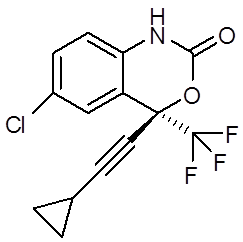
Fig.: Chemical structure of Efavirenz
Pages: 32-39 | 1774 Views 157 Downloads

How to cite this article:
P. Ravisankar, G. Mounika, CH. Devadasu, G. Devala Rao. Novel analytical method development and validation for the quantitative analysis of Efavirenz in bulk and pharmaceutical dosage forms by RP-HPLC. Pharma Innovation 2014;3(9):32-39.






