Toll Free Helpline (India): 1800 1234 070
Rest of World: +91-9810852116
Free Publication Certificate
Vol. 2, Issue 7 (2013)
Formulation and Evaluation of Lornoxicam Suppositories
Author(s):
Pushkar Baviskar, Shivkumar Jaiswal, Sayyad Sadique, Amol Landged
Pushkar Baviskar, Shivkumar Jaiswal, Sayyad Sadique, Amol Landged
Abstract:
Lornoxicam suppositories were prepared by using water soluble and oil soluble suppository bases. All the prepared suppositories were evaluated for various physical parameters like weight variation, drug content, hardness, Liqification time and temperature, disintegration and macro-melting range. In-vitro release study was performed USP type I apparatus (Basket type) using phosphate buffer pH 7.4 as dissolution media. The suppositories prepared were within permissible range of all physical parameters. In vitro drug released from water soluble bases (like PEG) was greater than that from oil soluble bases. Addition of HPMC, Glyceryl Behenate in agar suppositories to controlled release. The results suggest that of PEG of low molecular weight with high molecular weight in different percentage of release. The Sustained release suppositories can be prepared by addition of HPMC, Glyceryl Behenate in agar based suppositories and by use of bees wax in cocoa butter as base.
Lornoxicam suppositories were prepared by using water soluble and oil soluble suppository bases. All the prepared suppositories were evaluated for various physical parameters like weight variation, drug content, hardness, Liqification time and temperature, disintegration and macro-melting range. In-vitro release study was performed USP type I apparatus (Basket type) using phosphate buffer pH 7.4 as dissolution media. The suppositories prepared were within permissible range of all physical parameters. In vitro drug released from water soluble bases (like PEG) was greater than that from oil soluble bases. Addition of HPMC, Glyceryl Behenate in agar suppositories to controlled release. The results suggest that of PEG of low molecular weight with high molecular weight in different percentage of release. The Sustained release suppositories can be prepared by addition of HPMC, Glyceryl Behenate in agar based suppositories and by use of bees wax in cocoa butter as base.
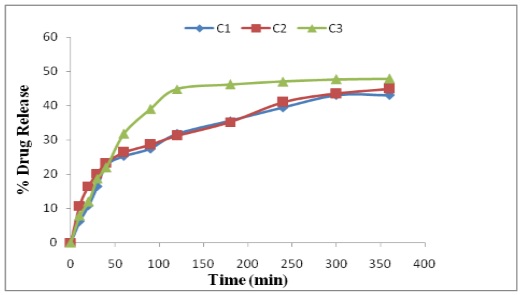
Fig.: In vitro release of lornoxicam from Cocoa butter based Suppositories
Pages: 20-27 | 2150 Views 495 Downloads

How to cite this article:
Pushkar Baviskar, Shivkumar Jaiswal, Sayyad Sadique, Amol Landged. Formulation and Evaluation of Lornoxicam Suppositories. Pharma Innovation 2013;2(7):20-27.






