Toll Free Helpline (India): 1800 1234 070
Rest of World: +91-9810852116
Free Publication Certificate
Vol. 2, Issue 3 (2013)
Post-Market In-Vitro Comparative Evaluation of Quality Control Parameters of Paracetamol Compressed Tablets Manufactured in Local Industrial Zones of Kpk Pakistan.
Author(s):
Zia-ur-Rahman, Imran khan, Alija Baig, Ali Raza Quraishi, Fazl e Zahir
Zia-ur-Rahman, Imran khan, Alija Baig, Ali Raza Quraishi, Fazl e Zahir
Abstract:
Paracetamol (acetaminophen) is an OTC drug available also in dosage form of compressed tablets in almost all countries, being extensively used as antipyretic and general analgesic. In KPK Pakistan numbers of brands are available manufactured in local industrial zones. Here in this laboratory research we have tried to follow the simple approaches i.e. the British Pharmacopoeia and USP and other standard book’s standards and procedures to evaluate the quality parameters of different brands (3 brands A, B and C), by selecting samples of different batches (3 batches of each), manufactured in industrial zones of KPK Pakistan (by 3 different manufacturers). Types of quality tests performed are weight variation, disintegration time, dissolution, hardness and friability. Finally the results evaluate no significant deviations from the compendial standards and specifications, and we got the aim of our study that these brands of local manufacturers in KPK are safe enough and could be used to achieve the desired therapeutic effect which has been discussed in paper.
Paracetamol (acetaminophen) is an OTC drug available also in dosage form of compressed tablets in almost all countries, being extensively used as antipyretic and general analgesic. In KPK Pakistan numbers of brands are available manufactured in local industrial zones. Here in this laboratory research we have tried to follow the simple approaches i.e. the British Pharmacopoeia and USP and other standard book’s standards and procedures to evaluate the quality parameters of different brands (3 brands A, B and C), by selecting samples of different batches (3 batches of each), manufactured in industrial zones of KPK Pakistan (by 3 different manufacturers). Types of quality tests performed are weight variation, disintegration time, dissolution, hardness and friability. Finally the results evaluate no significant deviations from the compendial standards and specifications, and we got the aim of our study that these brands of local manufacturers in KPK are safe enough and could be used to achieve the desired therapeutic effect which has been discussed in paper.
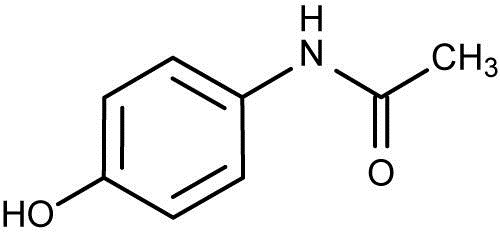
Fig.: fig 1
Pages: 11-15 | 2014 Views 223 Downloads

How to cite this article:
Zia-ur-Rahman, Imran khan, Alija Baig, Ali Raza Quraishi, Fazl e Zahir. Post-Market In-Vitro Comparative Evaluation of Quality Control Parameters of Paracetamol Compressed Tablets Manufactured in Local Industrial Zones of Kpk Pakistan.. Pharma Innovation 2013;2(3):11-15.






