Toll Free Helpline (India): 1800 1234 070
Rest of World: +91-9810852116
Free Publication Certificate
Vol. 3, Issue 11 (2015)
Second derivative spectrophotometric method development and validation for simultaneous estimation of gatifloxacin and prednisolone acetate in their combined dosage form
Author(s):
Prasanna Kumar Pradhan, Naimish Raiyani, Shreya R. Shah, Grishma H. Patel, Umesh Upadhyay
Prasanna Kumar Pradhan, Naimish Raiyani, Shreya R. Shah, Grishma H. Patel, Umesh Upadhyay
Abstract:
Gatifloxacin and Prednisolone Acetate combination is used for prevention and treatment of ophthalmic bacterial infections and inflammatory conditions. In Second Derivative Spectrophotometric method, Methanol is used as a mobile phase. linearity range obtained for the method were 1.5 – 4.5 μg/ml and 5 - 15 μg/ml with corresponding correlation coefficient of 0.9990 and 0.9994, for Gatifloxacin and Prednisolone Acetate respectively. Detection of Gatifloxacin and Prednisolone Acetate at 268 nm and 243 nm respectively. The method was found to be rapid, accurate and precise. This method was validated according to ICH guidelines.
Gatifloxacin and Prednisolone Acetate combination is used for prevention and treatment of ophthalmic bacterial infections and inflammatory conditions. In Second Derivative Spectrophotometric method, Methanol is used as a mobile phase. linearity range obtained for the method were 1.5 – 4.5 μg/ml and 5 - 15 μg/ml with corresponding correlation coefficient of 0.9990 and 0.9994, for Gatifloxacin and Prednisolone Acetate respectively. Detection of Gatifloxacin and Prednisolone Acetate at 268 nm and 243 nm respectively. The method was found to be rapid, accurate and precise. This method was validated according to ICH guidelines.
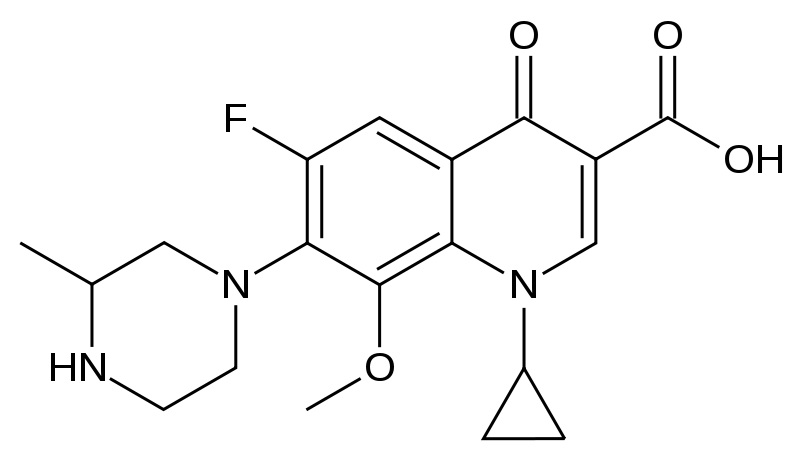
Fig.: Chemical structure of Gatifloxacin
Pages: 06-10 | 1796 Views 140 Downloads

How to cite this article:
Prasanna Kumar Pradhan, Naimish Raiyani, Shreya R. Shah, Grishma H. Patel, Umesh Upadhyay. Second derivative spectrophotometric method development and validation for simultaneous estimation of gatifloxacin and prednisolone acetate in their combined dosage form. Pharma Innovation 2015;3(11):06-10.







