Volume 3, Issue 4
Formulation and in-vitro evaluation of oral disintegrating tablets containing solid dispersions of Candesartan Cilexetil
Author: Ramya Krishna S.
Abstract: Candesartan Cilexetil is an angiotensin II receptor antagonist used mainly for the treatment of hypertension. The drug is having low solubility in biological fluids which results in poor bioavailability after oral administration. Hence present study was carried to enhance dissolution properties of candesartan cilexetil. Solid dispersions of candesartan cilexetil were prepared by using PEG-6000 as water soluble carries at various proportions 1:1, 1:2, 1:3. The kneading and solvent evaporation methods were used to prepare solid dispersions. The prepared dispersions were made into tablets by the direct compression method.
The release profile was studied in phosphate buffer pH 6.5 containing 0.35% polysorbate 20. It was found that the dissolution rate of tablets containing solid dispersions were higher than those of intact drug. The degree of dissolution rate enhancement depended on the amount of the carrier i.e., the higher the amount of the carrier used; the higher the dissolution rate was obtained. Among the prepared batches formulation F4 gave highest dissolution. The increase in the dissolution rate of the drug may be due to increase in wet ability, hydrophilic nature of the carrier and also possibility due to a reduction in drug crystalline.
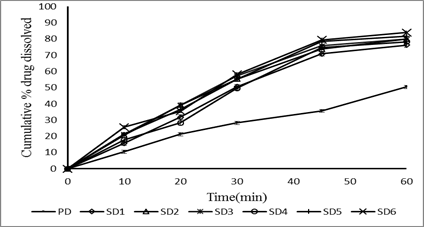
Fig: comparative in vitro release profile of solid dispersions prepared by kneading method.
Download Full Article: Click Here
Support Us
If you are interested in supporting our work and would like to contribute, you are welcome to mail me at jpbr.anil@gmail.com or at info@thepharmajournal.com it will be a great help and will surely be appreciated.

