Volume 2, Issue 3
Formulation and Evaluation of Rapidly Disintegrating Tablet of Ibuprofen
Author: Misra Shashi Kiran*, Himanshu Pandey
University Institute of Pharmacy, Chhatrapati Shahu Ji Maharaj University, Kanpur-208024, Uttar Pradesh, India.
Abstract: Objective: The aim of the proposed work was to formulate and characterize fast dissolving tablets of ibuprofen for rapid dissolution of drug and absorption, which may produce rapid onset of action. These dosage forms rapidly disintegrate and/or dissolve to release the drug as soon as they come in contact with saliva, thus obviating the need for water during administration, an attribute that makes them highly attractive for pediatric and geriatric patients.
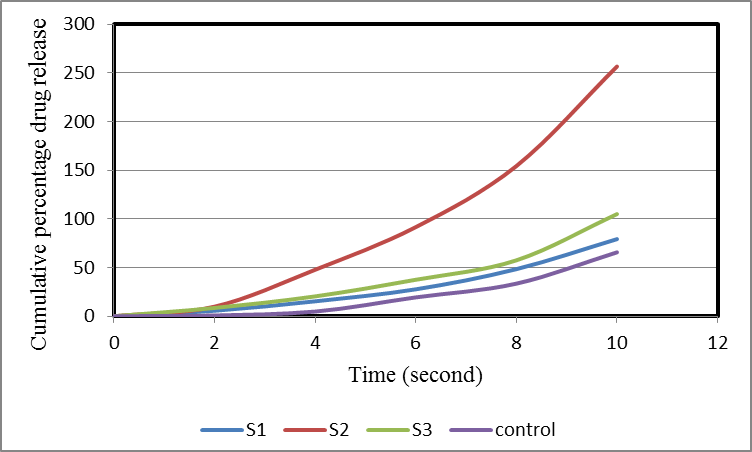
Method: The fast dissolving tablets of ibuprofen were prepared by two methods named melt technology and super disintegrant addition method. Comparatively evaluation parameters with respect to various physical parameters (weight variation, hardness, friability, etc.) were evaluated and drug release profile was analyzed. The concentration of sucrose and ac-di-sol were optimized for desired rapidly disintegrating formulations.
Result and conclusion: Both the methods of rapidly disintegrant tablets showed desirable results. The formulations prepared by super disintegrant addition were having lesser wetting time, disintegration time, in vitro dispersion time and better drug release as compared to the formulations prepared by melt technology.
Download Full Article: Click Here
Related Graphics:
Support Us
If you are interested in supporting our work and would like to contribute, you are welcome to mail me at jpbr.anil@gmail.com or at info@thepharmajournal.com it will be a great help and will surely be appreciated.

