Volume 2, Issue 3
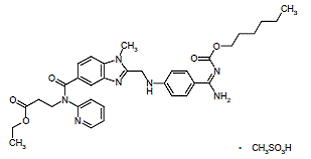
Dabigatran Etexilate: A Drug Update
Authors: Kamalpreet Kaur*, Dr. Vivek Gupta
Lovely Professional University, Jalandhar, Punjab- 144411, India.
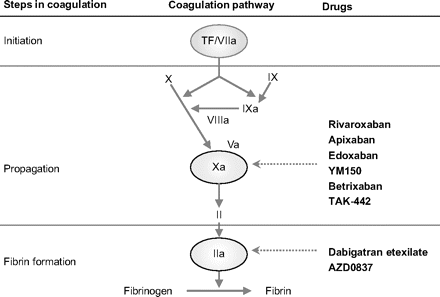
Abstract: The Direct Thrombin Inhibitors are a new class of anticoagulants that binds directly to the thrombin enzyme and blocks its effect. DTI’s prevents the conversion of fibrinogen to fibrin by binding the activity of thrombin. Pradaxa is used to help prevent strokes or serious blood clots in patients with atrial fibrillation. Dabigatran has shown efficacy in the prevention of thromboembolism in Phase 2 trials in orthopedic surgery and atrial fibrillation. Dabigatran has also undergone extensive Phase 3 clinical trials for the prevention of primary thromboembolism. FDA advisory committee recommends approval of Dabigatran Etexilate for prevention of Stroke in Atrial Fibrillation. Dabigatran has been licensed for the Total Hip Replacement and Total Knee Replacement in over 75 countries, including Europe and Canada. Hence, this paper reviews the existing safety and efficacy data for the use of dabigatran etexilate and discusses the potential role of dabigatran in the management of VTE, THR, TKR, atrial fibrillation.
Download Full Article: Click Here
Related Graphics:
Support Us
If you are interested in supporting our work and would like to contribute, you are welcome to mail me at jpbr.anil@gmail.com or at info@thepharmajournal.com it will be a great help and will surely be appreciated.

