Toll Free Helpline (India): 1800 1234 070
Rest of World: +91-9810852116
Free Publication Certificate
Vol. 2, Issue 3 (2013)
Formulation and Evaluation of Diphenhydramine Hcl Rapid Release Gelcaps 25 Mg
Author(s):
Margret chandira, A. Pasupati, H.V. Adinarayana innamuri, M. Komala, N.K. Agarwal, Debjit Bhowmik
Margret chandira, A. Pasupati, H.V. Adinarayana innamuri, M. Komala, N.K. Agarwal, Debjit Bhowmik
Abstract:
The objective of the present study was to develop Rapid Release Gelcaps of Diphenhydramine HCl, for treatment of Allergic symptoms and irritant cough. The Rapid Release gelcaps were prepared by Direct compression method using Pregelatinised maize starch and croscarmellose sodium in various concentrations. The granules showed satisfactory flow properties and compressibility. All the 8 formulations showed acceptable pharmacopoeial standards. The result of formulation B8 (40 mg Pregelatinised maise starch and 12 mg Croscarmellose sodium) Rapid Release of Diphenhydramine HCl. Successful formulation was found stable after evaluation for physicochemical parameters when kept for 30 days at room temperature, 40±2 °C & 75%RH and 2-8 °C. It concluded that gelcaps containing Diphenhydramine HCl (40 mg Pregelatinised maise starch and 12 mg Croscarmellose sodium) provide a better option for Rapid release of drug.
The objective of the present study was to develop Rapid Release Gelcaps of Diphenhydramine HCl, for treatment of Allergic symptoms and irritant cough. The Rapid Release gelcaps were prepared by Direct compression method using Pregelatinised maize starch and croscarmellose sodium in various concentrations. The granules showed satisfactory flow properties and compressibility. All the 8 formulations showed acceptable pharmacopoeial standards. The result of formulation B8 (40 mg Pregelatinised maise starch and 12 mg Croscarmellose sodium) Rapid Release of Diphenhydramine HCl. Successful formulation was found stable after evaluation for physicochemical parameters when kept for 30 days at room temperature, 40±2 °C & 75%RH and 2-8 °C. It concluded that gelcaps containing Diphenhydramine HCl (40 mg Pregelatinised maise starch and 12 mg Croscarmellose sodium) provide a better option for Rapid release of drug.
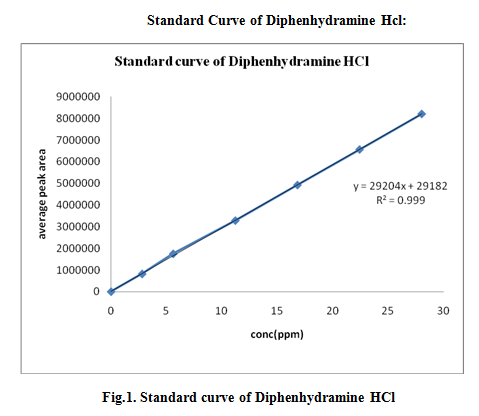
Fig.: Standard curve of Diphenhydramine HCL
Pages: 01-10 | 2368 Views 510 Downloads

How to cite this article:
Margret chandira, A. Pasupati, H.V. Adinarayana innamuri, M. Komala, N.K. Agarwal, Debjit Bhowmik. Formulation and Evaluation of Diphenhydramine Hcl Rapid Release Gelcaps 25 Mg. Pharma Innovation 2013;2(3):01-10.







