Volume 4, Issue 1
Simultaneous estimation method development as analytical method for flupentixol dihydrochloride and melitracen hydrochloride from their combine pharmaceutical dosage forms by RP-HPLC
Author: Akhil Nagar, Naresh N. Chugh
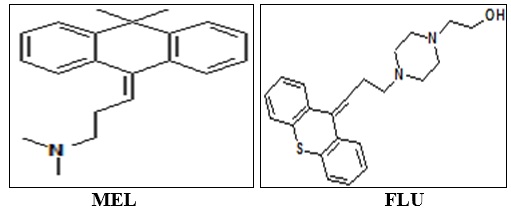
Abstract: A simple, precise and accurate method has been described for the estimation of Flupentixol and Melitracen in the formulation using SP Thermo Separation Products HPLC system and Thermo scientific BDS C8 column (150×4.6 mm) in isocratic mode consisting of LC-10AT pumps and UV detector with mobile phase potassium dihydrogen phosphate buffer: methanol: ACN (3:6:1), at flow rate of 1.5 ml/min. The effluent is monitored at 230 nm. The retention times of Melitracen and Flupentixol were 3.16 and 5.31 min respectively. The linearity range for Melitracen and Flupentixol were found to be 80- 120 µg/ml. The correlation co-efficient were closed in 1 proving the good linearity between the concentration of drug and response. The % RSD values of 3 precision were less than 2, which indicate that the method has good reproducibility. The method validation parameters like theoretical plates, resolution, tailing factor, LOD and LOQ were found to be within the USP standards. As the chromatogram for Melitracen and Flupentixol in formulation is free from any other peaks except at the retention time corresponding to drugs, it was revealed that excipients used in the formulation were not interfering in the method. Thus the proposed method is suitable for routine analysis, formulations containing of Melitracen and Flupentixol.
Download Full Article: Click Here
Support Us
If you are interested in supporting our work and would like to contribute, you are welcome to mail me at jpbr.anil@gmail.com or at info@thepharmajournal.com it will be a great help and will surely be appreciated.

