Volume 2, Issue 7
Development and Validation of A Rp-Hplc Method for The Simultaneous Determination of Luteolin and Apigenin in Herb of Achillea millefolium L.
Author: Andriy Gudzenko 1*
1. State Laboratory for Quality Control of Medicines, State Institution “Institute of Pharmacology and Toxicology of National Medical Academy of Science of Ukraine”, Kiyv, Ukraine.
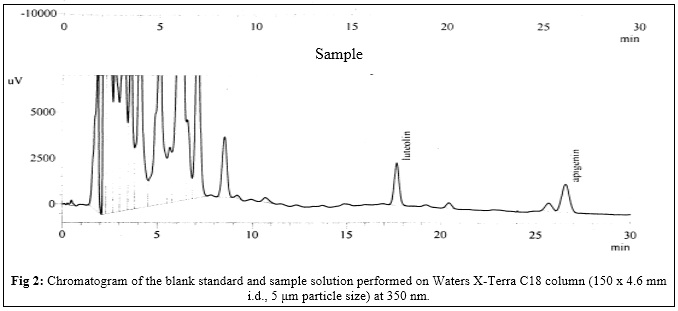
Abstract: A method for separation and quantification of two flavonoids by reverse-phase high performance liquid chromatography (HPLC) was developed and validated. Flavonoids present in herb of Achillea millefolium L. were analyzed. Luteolin and apigenin were used as calibration standards. The analysis was performed using a Waters X-Terra C18 column (250 x 4.6 mm i.d., 5 μm particle size), as stationary phase, with a flow rate of 1 mL/min and detection at a wavelength of 350 nm. The proposed method was validated by ICH Harmonised Tripartite Guidelines “Validation of analytical procedures: Text and Methodology Q2 (R1)”. In this study, an excellent linearity was obtained with r higher than 0.99. Besides, the chromatographic peaks showed good resolution. With other validation data, including precision, specificity, accuracy and robustness, this method demonstrated good reliability and sensitivity, and can be conveniently used for the quantification of luteolin and apigenin in in herb of Achillea millefolium L. Further this method can be applied to a standardization of multicomponent herbal remedies, that incorporate Achillea millefolium L.
Download Full Article: Click Here
Support Us
If you are interested in supporting our work and would like to contribute, you are welcome to mail me at jpbr.anil@gmail.com or at info@thepharmajournal.com it will be a great help and will surely be appreciated.

