Volume 2, Issue 7
Formulation and Evaluation of Lornoxicam Suppositories
Author: Pushkar Baviskar 1*, Shivkumar Jaiswal 2, Sayyad Sadique 3, Amol Landged 4
1. S.M.B.T College of Pharmacy, Nandi hills Dhamangaon, Igatpuri, Nashik, (M.S.) 422403.
2. S.M.B.T College of Pharmacy, Nandi hills Dhamangaon, Igatpuri, Nashik, (M.S.) 422403.
3. Amrutvahini College of pharmacy, Sangamner, (M.S.) 422608.
4. Amrutvahini College of pharmacy, Sangamner, (M.S.) 422608.
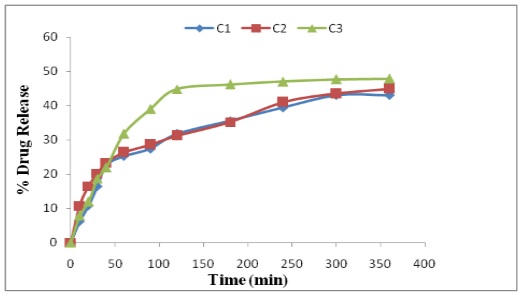
Abstract: Lornoxicam suppositories were prepared by using water soluble and oil soluble suppository bases. All the prepared suppositories were evaluated for various physical parameters like weight variation, drug content, hardness, Liqification time and temperature, disintegration and macro-melting range. In-vitro release study was performed USP type I apparatus (Basket type) using phosphate buffer pH 7.4 as dissolution media. The suppositories prepared were within permissible range of all physical parameters. In vitro drug released from water soluble bases (like PEG) was greater than that from oil soluble bases. Addition of HPMC, Glyceryl Behenate in agar suppositories to controlled release. The results suggest that of PEG of low molecular weight with high molecular weight in different percentage of release. The Sustained release suppositories can be prepared by addition of HPMC, Glyceryl Behenate in agar based suppositories and by use of bees wax in cocoa butter as base.
Download Full Article: Click Here
Support Us
If you are interested in supporting our work and would like to contribute, you are welcome to mail me at jpbr.anil@gmail.com or at info@thepharmajournal.com it will be a great help and will surely be appreciated.

