Toll Free Helpline (India): 1800 1234 070
Rest of World: +91-9810852116
Free Publication Certificate
Vol. 2, Issue 4 (2013)
Validated Spectrophotometric Methods for Simultaneous Estimation of Sildenafil Citrate and Dapoxetine Hcl in Tablet Dosage Form
Author(s):
Albin Pt, Y Haribabu, Sosamma Cicy Eapen, Sheeja Velayudhan Kutty, Kumar P, Nithyamol P.
Albin Pt, Y Haribabu, Sosamma Cicy Eapen, Sheeja Velayudhan Kutty, Kumar P, Nithyamol P.
Abstract:
Two simple UV-Spectrophotometric methods have been developed for simultaneous determination of sildenafil citrate and dapoxetine HCl in pharmaceutical formulation. For both the methods stock solutions were prepared in methanol followed by the further required dilutions with methanol. Proposed Vierodt’s method and double point standardization method, the ï¬max for the estimation of sildenafil citrate and dapoxetine HCl were selected at 292nm and 231nm respectively. In both methods, the linearity range lies between 2-20 µg/mL for sildenafil citrate and 1.2-12 µg/mL for dapoxetine HCl at their respective wavelengths. By Vierodt’s method the percentage of sildenafil citrate and dapoxetineHCl was found to be 99.25%, dapoxetine HCl 99.08% respectively. It was estimated to be 98.1% for sildenafil Citrate and 99.16% for Ddapoxetine HCl by double point standardization method. Both these methods were found to be accurate, precise, stable and robust as indicated by low values of % RSD. Thus the present study gives excellent method for the determination of both the drugs in combined tablet formulation.
Two simple UV-Spectrophotometric methods have been developed for simultaneous determination of sildenafil citrate and dapoxetine HCl in pharmaceutical formulation. For both the methods stock solutions were prepared in methanol followed by the further required dilutions with methanol. Proposed Vierodt’s method and double point standardization method, the ï¬max for the estimation of sildenafil citrate and dapoxetine HCl were selected at 292nm and 231nm respectively. In both methods, the linearity range lies between 2-20 µg/mL for sildenafil citrate and 1.2-12 µg/mL for dapoxetine HCl at their respective wavelengths. By Vierodt’s method the percentage of sildenafil citrate and dapoxetineHCl was found to be 99.25%, dapoxetine HCl 99.08% respectively. It was estimated to be 98.1% for sildenafil Citrate and 99.16% for Ddapoxetine HCl by double point standardization method. Both these methods were found to be accurate, precise, stable and robust as indicated by low values of % RSD. Thus the present study gives excellent method for the determination of both the drugs in combined tablet formulation.
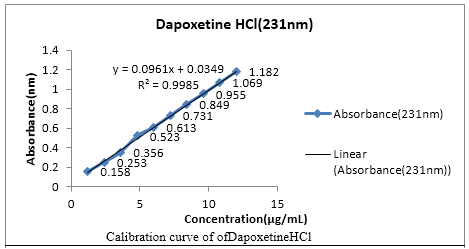
Fig.: fig 1
Pages: 40-45 | 1841 Views 153 Downloads

How to cite this article:
Albin Pt, Y Haribabu, Sosamma Cicy Eapen, Sheeja Velayudhan Kutty, Kumar P, Nithyamol P.. Validated Spectrophotometric Methods for Simultaneous Estimation of Sildenafil Citrate and Dapoxetine Hcl in Tablet Dosage Form. Pharma Innovation 2013;2(4):40-45.







